Aluminium »
PDB 1a6e-1tx4 »
1tuy »
Aluminium in PDB 1tuy: Acetate Kinase Complexed with Adp, ALF3 and Acetate
Enzymatic activity of Acetate Kinase Complexed with Adp, ALF3 and Acetate
All present enzymatic activity of Acetate Kinase Complexed with Adp, ALF3 and Acetate:
2.7.2.1;
2.7.2.1;
Protein crystallography data
The structure of Acetate Kinase Complexed with Adp, ALF3 and Acetate, PDB code: 1tuy
was solved by
A.Gorrell,
S.H.Lawrence,
J.G.Ferry,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 28.43 / 3.00 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 180.043, 67.196, 82.142, 90.00, 103.13, 90.00 |
| R / Rfree (%) | 20.1 / 26.3 |
Other elements in 1tuy:
The structure of Acetate Kinase Complexed with Adp, ALF3 and Acetate also contains other interesting chemical elements:
| Fluorine | (F) | 3 atoms |
Aluminium Binding Sites:
The binding sites of Aluminium atom in the Acetate Kinase Complexed with Adp, ALF3 and Acetate
(pdb code 1tuy). This binding sites where shown within
5.0 Angstroms radius around Aluminium atom.
In total only one binding site of Aluminium was determined in the Acetate Kinase Complexed with Adp, ALF3 and Acetate, PDB code: 1tuy:
In total only one binding site of Aluminium was determined in the Acetate Kinase Complexed with Adp, ALF3 and Acetate, PDB code: 1tuy:
Aluminium binding site 1 out of 1 in 1tuy
Go back to
Aluminium binding site 1 out
of 1 in the Acetate Kinase Complexed with Adp, ALF3 and Acetate
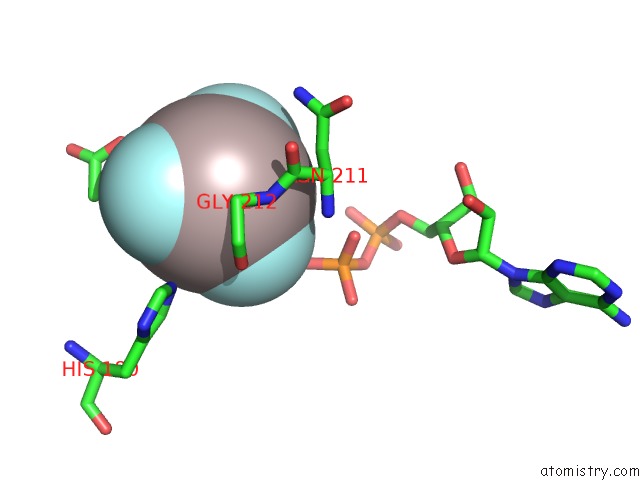
Mono view
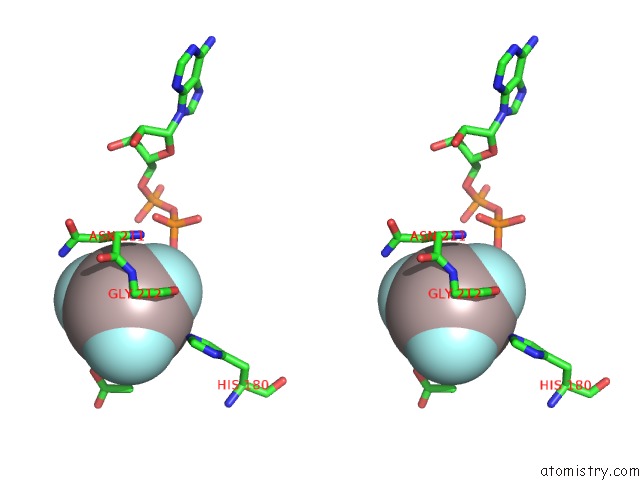
Stereo pair view

Mono view

Stereo pair view
A full contact list of Aluminium with other atoms in the Al binding
site number 1 of Acetate Kinase Complexed with Adp, ALF3 and Acetate within 5.0Å range:
|
Reference:
A.Gorrell,
S.H.Lawrence,
J.G.Ferry.
Structural and Kinetic Analyses of Arginine Residues in the Active Site of the Acetate Kinase From Methanosarcina Thermophila. J.Biol.Chem. V. 280 10731 2005.
ISSN: ISSN 0021-9258
PubMed: 15647264
DOI: 10.1074/JBC.M412118200
Page generated: Sun Jul 6 21:38:20 2025
ISSN: ISSN 0021-9258
PubMed: 15647264
DOI: 10.1074/JBC.M412118200
Last articles
Cl in 7SFLCl in 7SBC
Cl in 7SF6
Cl in 7SFI
Cl in 7SF3
Cl in 7SBJ
Cl in 7SEL
Cl in 7SET
Cl in 7SDR
Cl in 7SCI