Aluminium »
PDB 2xzl-3wgu »
2zjy »
Aluminium in PDB 2zjy: Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp
Enzymatic activity of Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp
All present enzymatic activity of Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp:
3.6.5.1;
3.6.5.1;
Protein crystallography data
The structure of Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp, PDB code: 2zjy
was solved by
T.Morikawa,
A.Muroya,
S.Sugio,
K.Wakamatsu,
T.Kohno,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 28.78 / 2.80 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 79.375, 79.375, 105.297, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.1 / 26 |
Other elements in 2zjy:
The structure of Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp also contains other interesting chemical elements:
| Fluorine | (F) | 4 atoms |
| Magnesium | (Mg) | 1 atom |
Aluminium Binding Sites:
The binding sites of Aluminium atom in the Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp
(pdb code 2zjy). This binding sites where shown within
5.0 Angstroms radius around Aluminium atom.
In total only one binding site of Aluminium was determined in the Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp, PDB code: 2zjy:
In total only one binding site of Aluminium was determined in the Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp, PDB code: 2zjy:
Aluminium binding site 1 out of 1 in 2zjy
Go back to
Aluminium binding site 1 out
of 1 in the Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp
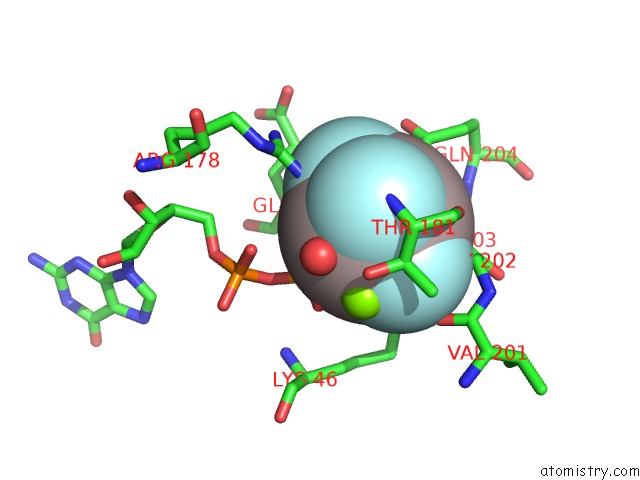
Mono view
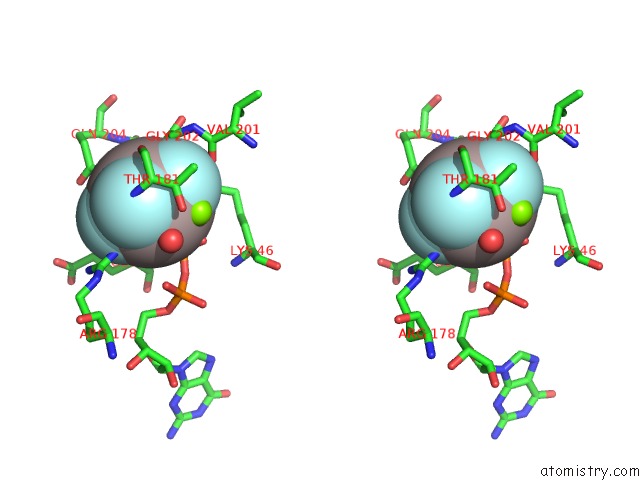
Stereo pair view

Mono view

Stereo pair view
A full contact list of Aluminium with other atoms in the Al binding
site number 1 of Structure of the K349P Mutant of Gi Alpha 1 Subunit Bound to ALF4 and Gdp within 5.0Å range:
|
Reference:
T.Morikawa,
A.Muroya,
S.Sugio,
K.Wakamatsu,
T.Kohno.
How Gpcrs Activate G Proteins: Structural Changes Form C-Terminal Tail to Gdp Binding Pocket To Be Published.
Page generated: Wed Jul 10 09:34:20 2024
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF